Add:No. 369 Hedong Rd. Hi-tech Industrial Development Zone, 266112, Qingdao, Shandong, China
E-mail:sales@hightopbio.com

to Use

Reaction

Accuracy
The SARS-Cov-2 and iniluenza A/B Antieen Rapid Test is intended orin vitro qualtative detection to severe acute respiratorsyndrome coronavirus 2 (SARS-CoV-2) antigen and influenza A/8 (fLUA/B) antigen in human nasopharyngeal swab ororopharyngeal swab samples.
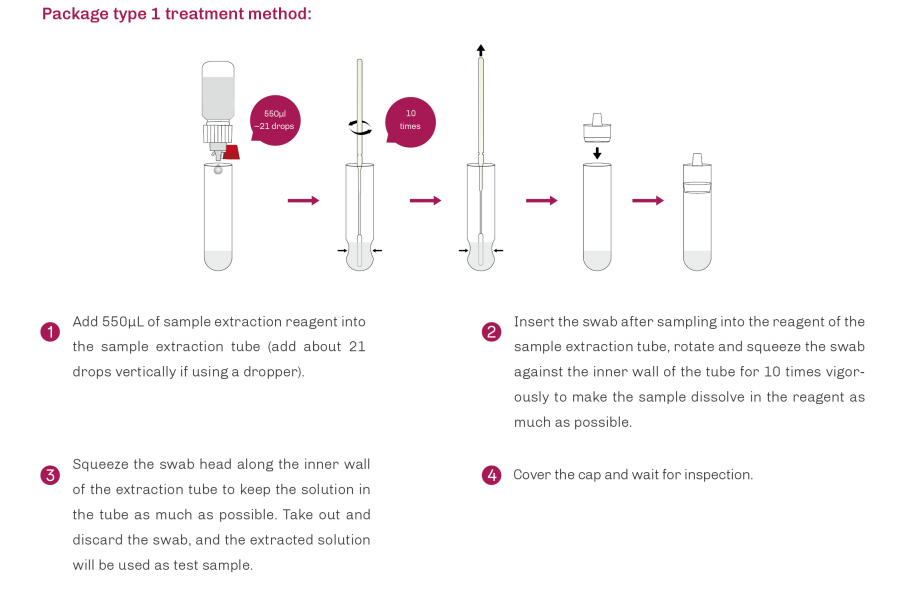
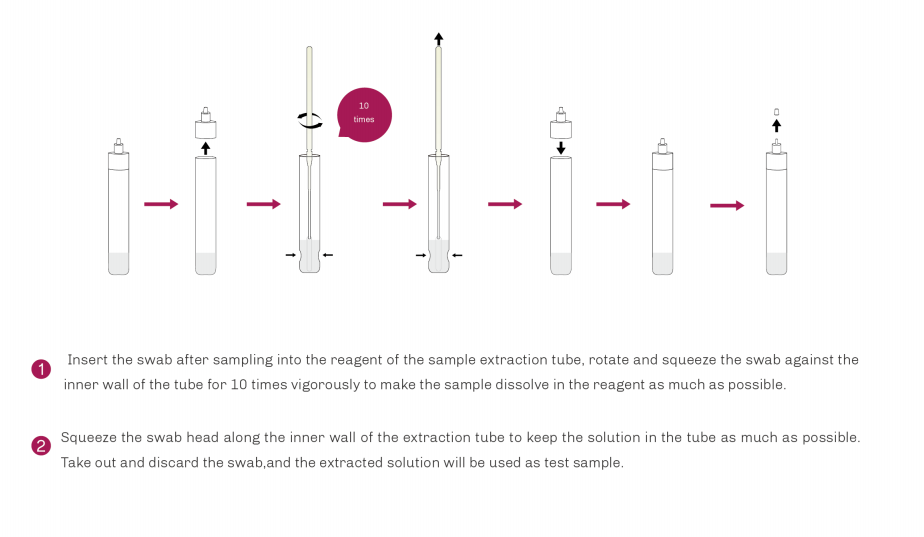
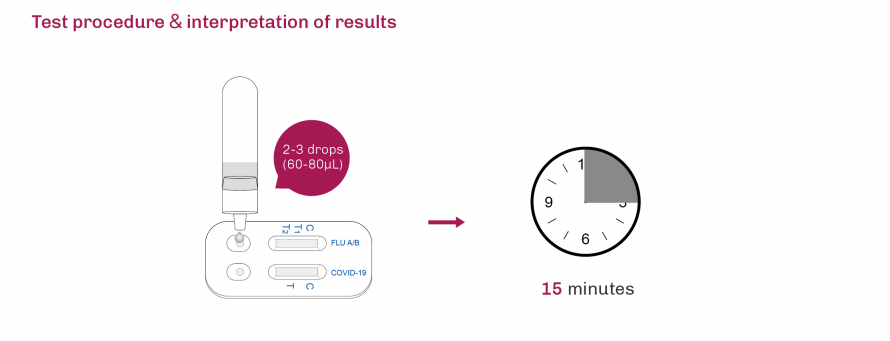
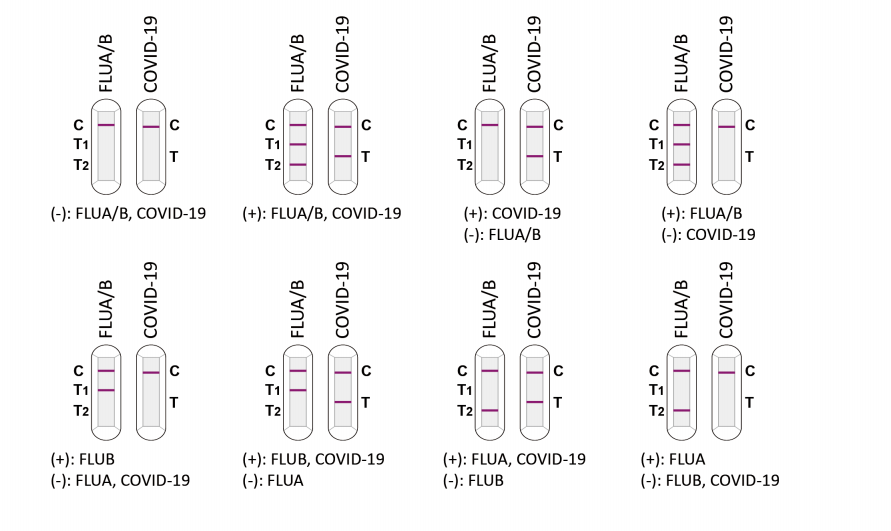
| Product Description | Format | Sample Type | Packing | Validity | LoD | Certification |
SARS-CoV-2/Influenza A+B Antigen Rapid Test | Cassette | Nasopharyngeal swa Oropharyngeal swab | 20T | 24 Months | SARS-CoV-2: 8 TCID50/L Flu A: 3.2×104TCID50/L Flu B: 5.2×105TCID50/L | CE |
