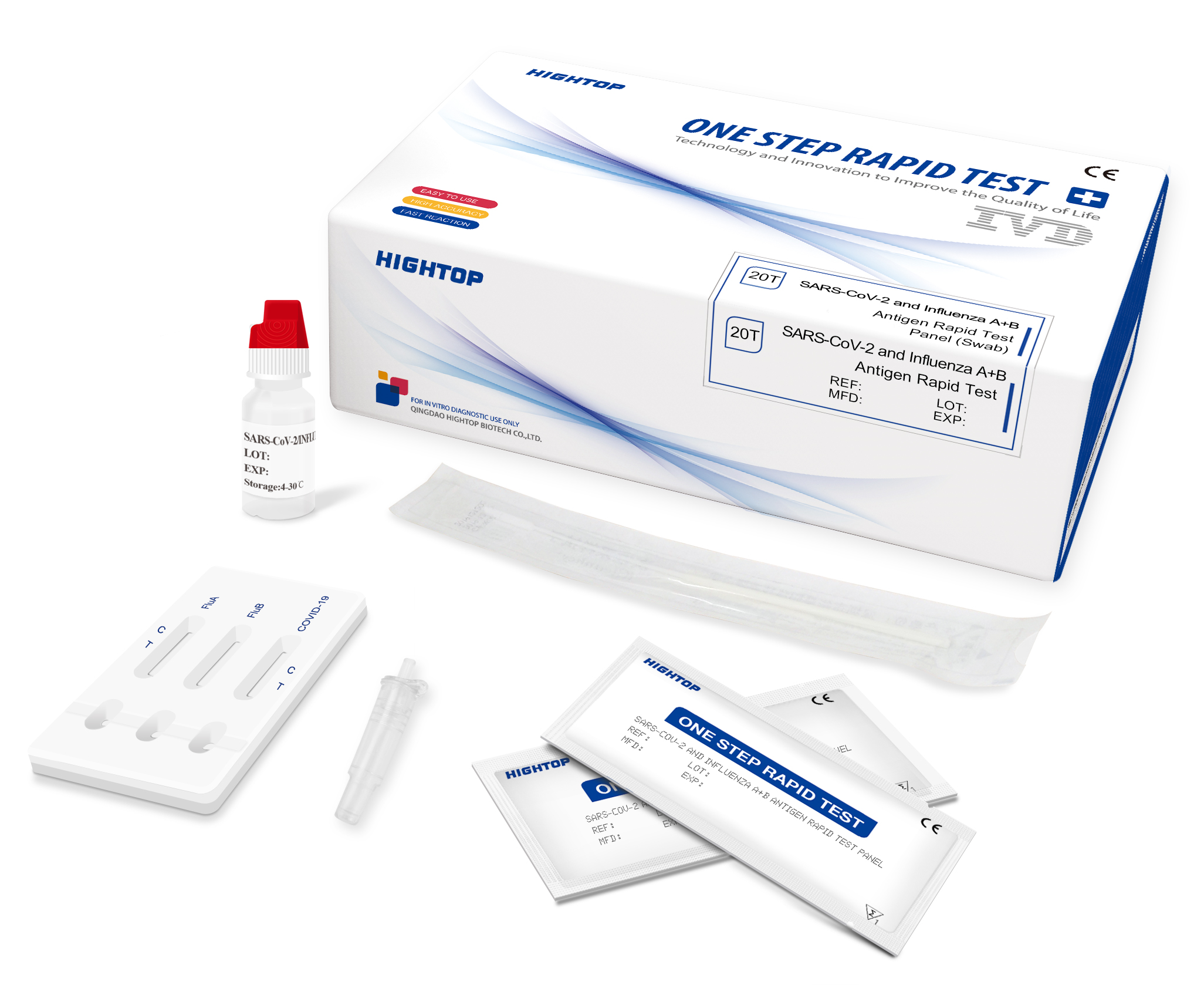


SARS-CoV-2 and Influenza A+B Antigen Rapid Test
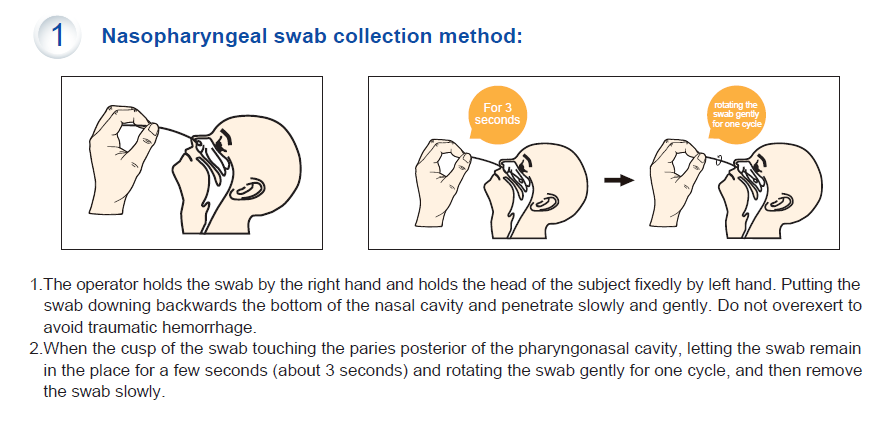
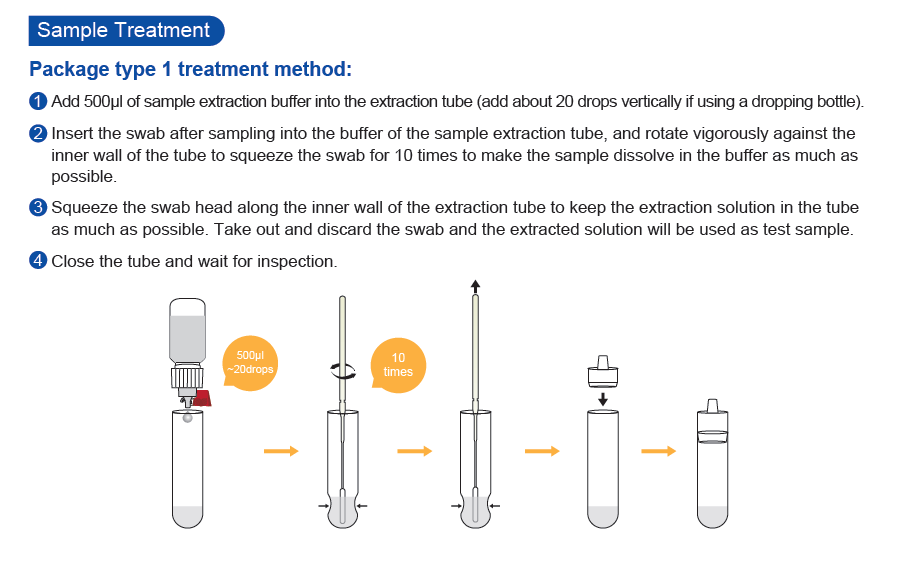
- Specimen: Nasopharyngeal Swab or Oropharyngeal Swab
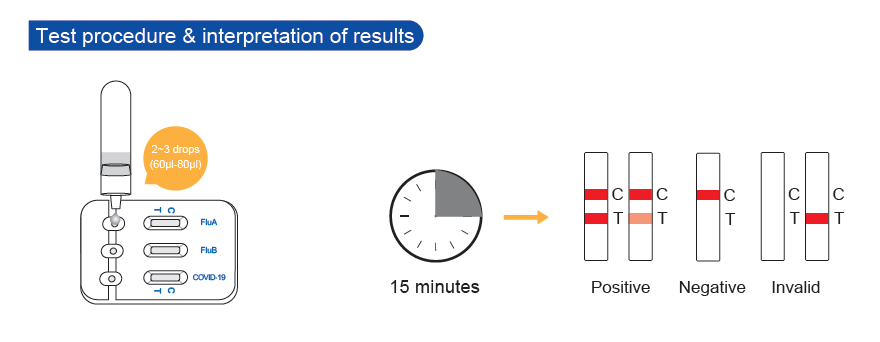
- Fast Reaction: 15 minutes
SARS-CoV-2 Ag
- Sensitivity(PPA): 95.00% (95% CI: 88.83%-97.85%)
- Specificity(NPA): 99.75% (95% CI: 98.60%-99.96%)
- Total Consistent(OPA): 98.80% (95% CI: 97.41%-99.45%)
The SARS-CoV-2 and Influenza A+B Antigen Rapid Test is intended for in vitro qualitative detection of SARS-CoV-2, influenza A virus, influenza B virus antigen in human nasopharyngeal swab or oropharyngeal swab samples.It it to be used as an aid in the diagnosis of coronavirus infection disease (COVID-19), which is caused by SARS-CoV-2.
The test provides preliminary test results. Negative results don't preclude SARS-CoV-2 infection and they cannot be used as the sole basis for treatment or other management decision.
COVID-19, it is a novel coronavirus with new strain thathas not been previously identified in humans, belongs to Coronaviruses (CoV) suchas Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome(SARS-CoV).
| Product Name | Specimen | Kit Size | Status |
SARS-CoV-2 and Influenza A+B Antigen Rapid Test | Nasopharyngeal Swab / Oropharyngeal Swab | 20 Tests/box, 48 Boxes/ctn | CE |
Please contact us for any enquiries.
Email: sales@hightopbio.com